http://gorila.netlab.cz/coins/jan-bukvic-cisteni-a-konzervace-minci-cz.pdf
:-)
Coin cleaning and preservation part.1
Categories: Minting - Numismatics
Hunters and other otherwise "handicapped" colleagues, I am a complete novice in this field, but when I get excited about something, then I try to get and also pass on as much information as possible. After the very first time I kicked and removed a coin from the ground, I knew I would have to do some cleaning and not just any cleaning. For this reason, I started looking for information on what to do and how to do it. And you know that every "hunter" has a different opinion.
I would like to offer you all a series of articles that discusses cleaning and preserving coins from all sides. The material is not off the top of my head, just edited to provide a comprehensive guide for all of us.
Each of us will take our own take from it, and of course it is not a dogma to be followed, just a suggested path, so please don't stone me. I will deliberately list the cleaning chemical baths after the coins we find most often, so that you don't become amateur chemists before you at least have an idea what to use the baths for.
The series will consist of 9 parts:
1) Introduction, Hardness and Density of Material
2) General Basics of Coin Cleaning
3) Copper
4) CleaningBaths
5) Gold + Silver
6) Nickel + Zinc
7) Iron + Aluminum
8) Tin + Lead
9) Preservation
INTRODUCTION
With information about the deteriorating environment increasing daily, environmental problems are becoming more and more of a concern to mankind. The aim is to provide as healthy an environment as possible for humans, for living nature.
Unfortunately, even the numismatic hunter is increasingly discovering that even his collection will not remain safe from the harmful effects of the environment unless he does something about it. What used to take centuries is now decades, sometimes even less. Just take a look at the museum's coin displays. The silver coins on display often look more like tin or lead.
Newly issued silver jubilee coins look new for at most a year. Oxidation spots, especially on coins of lesser silver, are becoming increasingly common. Base metal coins, of course, are no better off.
Previously, coins remained for decades, whether in a hoard or on display, with virtually no visible change. Today, perhaps only gold coins can successfully counteract the deteriorating environment. However, these usually represent, in terms of number of pieces, an insignificant part of the collection.
And so, after a while, every coin and medal collector inevitably comes to the decision to clean and preserve his or her collecting material. Since these are often valuable items that could be damaged or even devalued by unprofessional intervention, he seeks guidance from specialist literature.
Over the past 15 to 20 years, chemistry has advanced considerably, and the knowledge and experience of numismatic hunters has deepened as well. We have tried to reflect all this in the individual chapters. We will therefore get acquainted with not only classical but also modern methods.
It should be noted at the outset, however, that the opinion on some of the methods used is not uniform even among experts. Nohejlová in Fundamentals of Numismatics states, among other things, that "in one place a method is still used which is condemned in another place".
In this handbook, we list only those methods that we ourselves have tried with success. If more than one way leads to the goal, we have given preference to the one that is more feasible even for the layman and non-chemist, if possible by his own efforts and available means.It goes without saying that all identification, cleaning and conservation work will be carried out by each person on his own their own risk, but as long as the procedures laid down are followed, there is no danger to the numismatic material or to themselves.
We have tried not to go into technical details, the most necessary theory is only where it is necessary to explain certain changes on the coins, or to justify a certain procedure, etc.
In addition to coins, the methods given in the manual apply in full to medals and other numismatic material such as tokens, various metal stamps, etc., However, it is absolutely necessary to know the composition of the metal from which the object is made before starting work. In the case of coins, we can usually get the necessary information from a catalogue, while in the case of medals, experience and a feel for the material will often be our guide. Nevertheless, it sometimes happens that we are in doubt as to what metal or alloy it is. We have no choice but to try to determine it.
In the case of numismatic material, however, we must rule out in advance the methods commonly employed in jewellery, which as a rule consist...of filing the object and then testing it with acids or other substances.
Therefore, in the chapter on density, we present a simple, though still little used, method, the so-called. We can, in most cases, determine the metal in question by means of a method which is not very useful and which leaves no trace on the object under examination.Medals made of precious metals are usually hallmarked. In order to be able to correctly determine the purity, the manual is supplemented with hallmarks, not only Czechoslovak and Austro-Hungarianbut also hallmarks of some neighbouring countries, whose numismatic monuments a Czechoslovak collector may encounter.
And because it may happen that despite all our efforts we will not be able to identify the object in question more closely and we will be referred to the expertise of someone more experienced, or directly to someprofessional workplace, we are also including a new simple guide for quick and easy making of a perfect impression, although this issue is only marginally related to the focus of the manual. But it is always incomparably more profitable to risk damage or even loss of a practically worthless impression than of a possible numismatic rarity.
The method of storage is also of considerable importance for numismatic material, so we will briefly describe this circumstance as well.
And before we get down to work, let us keep in mind that it is usually not enough to simply read the instructions; the procedures must be mastered and the subject matter must be penetrated. Only then will the result of our efforts be fully satisfactory.
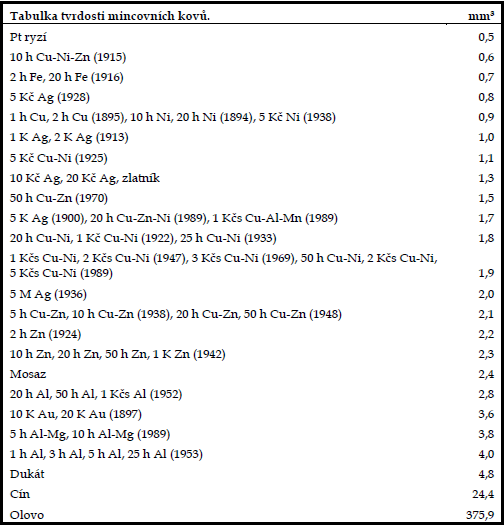
HARDNESS OF THE COIN MATERIAL
An important characteristic of coin metals is their hardness. The higher the hardness, the less wear and tear the coins will experience in circulation.
But hardness is also important when cleaning coins, as it influences the choice of mechanical cleaning tools. The hardness of metals can be tested in several different ways, each of which corresponds to a specific scale.
For our purposes, however, the hardness of individual pure metals is insufficient for two reasons. Firstly, the coinage is dominated by alloys for which it is difficult to express the hardness, and so the relevant tables do not even usually give it, and in the case of pure metals, the general hardness indication is usually different from the actual hardness of the coin minted from it.
The difference is explained by the fact that the material is significantly compacted during minting, so that the struck coin is harder than the casing before minting. The fact that the change in hardness is also due to the heat treatment of the casing during minting is not negligible. Heating the casing softens it, making it easier and more perfect to mint, and after cooling it returns to its original hardness, even surpassing it in some materials over time.
And so it proves most practical from a numismatic point of view to know the hardness of individual real coins.
This has been done by modifying Brinell's method. We used a steel ball, which was pressed into the coin field of each coin with a pressing force of 4000 N. The size of the steel ball and the pressing force remained constant throughout the tests.
As a result, different sized pits, geometrically called spherical sections, were pressed into the test coins. The larger the pit, the softer the metal. We measured the diameters of the individual spherical sections with a Brinell magnifying glass, plugged them into the appropriate formula and calculated the volume of the extruded pits for each coin in mm3.
In all cases, we used real coins and in particular the complete crown currency from 1892 to the present. Only in the case of platinum, for obvious reasons, we tried a pure platinum sheet of 1.5 mm thickness instead of a coin, and in the case of tin and lead we used medals.
For our purpose it is very important to know the hardness of the brass from which the brass brush wires, occasionally used by numismatists, are made. In connection with the coins, we therefore carried out a test of the hardness of this material by the same procedure.
A table compiled from the results gives us an overview of the relative hardness of the various coin metals. We may even be surprised when we become more closely acquainted with this table; for some cases we may even be hard pressed to find a theoretical explanation. We are struck, for example, by the fact that silver coins of the same purity have different hardness. Here we must be content with the observation that these are coins from different periods and mints, each mint having its own technology, differing differing only by fractions of the percentage of impurities used and the method of heat treatment, which, however, have a bearing on the final hardness of the coin.
However, experimental tests are decisive and have repeatedly confirmed the position of each coin in the table.
On the other hand, it is probably not surprising that the table begins with platinum, which is the hardest, and that it concludes with lead, which is by far the softest.
In addition to the general orientation, which is undoubtedly important for any numismatic hunter, the table of hardness of coin metals thus gives us a clue as to when we can consider using a brass brush when cleaning coins.
Although there are many metals harder than brass listed in the table, this by no means means means that we should mindlessly clean one coin at a time with a brass brush.
There is one important circumstance that we must first consider. There is a patina on practically every coin, even if it is sometimes almost imperceptible, just a tinge that we are not even aware of. And it is on the patina that the use of a brass brush can result in unpleasant scratches, because the patina is usually softer than the base metal, on which it doesn't adhere firmly enough, and it's a different, usually darker colour. Thus, even if the brass wires do not damage the base metal, the coin may look scratched.
We will therefore always consider carefully before deciding to use a brass brush. As a rule, this will be after all other options have been exhausted and the coins to be cleaned will be among the more common ones, so mostly made of iron, zinc, copper, cupro-nickel and nickel.However, we need not be panic-stricken about using a brass brush. It is enough, if we follow our senses and act sensibly, and we will soon see that the exclamation marks that often accompany the use of a metal toothbrush are exaggerated.
The situation is different, of course, when it comes to a steel brush, which we avoid by a wide margin. The damage caused would be truly irreparable. But let us trust that even a beginner is already instructed in this respect.
But as for the brass brush, we shall soon find that there are cases in which it is irreplaceable. And most of the time we will be pleasantly surprised at the result.
Let us perhaps add that brass brushes have a diameter of the wires used of either 0.15 mm or 0.2 mm. If we have the option, we opt for weaker, finer wires.
DENSITY OF MINING MET ALS
Density is an important physical quantity that gives us the mass of a volume of 1 m3 of a given substance in kilograms, unit kg/m3.In the literature, especially older literature, one comes across equivalent terms such as specific gravity, specific gravity, etc., usually expressed in more convenient units (g/cm3 or kg/dm3), which, when expressed in this way but dimensionlessly, also give the so-called 'weight'. relative mass, expressing how many times greater or lesser the mass of a given láthan the mass of the same volume of distilled water at 4° C. (at this temperature the density of water is maximum and is exactly 1 kg/dm3).
Density is one of the properties that characterise pure substances, i.e. chemically uniquely defined elements or compounds.A certain value of density therefore corresponds, for example, also to individual pure metals. However, in the case of alloys, the density cannot be precisely determined in advance, even if we know the exact composition and densities of the individual components, so the density values of technically important substances and chemical individuals are often tabulated.
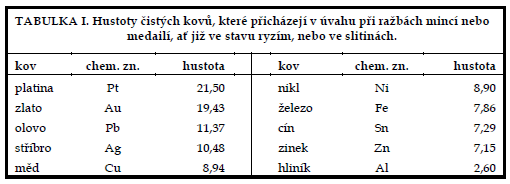
From the point of view of numismatics, it is important for us to know the density values of the coin metals, so we tabulate them in this chapter. These data will facilitate our decision whether, for example, the medal under examination is gold or only gold-plated, whether the newly minted four-ducats of Franz Joseph I. is really of the purity 986/1000 and not 900, 750 or even 585/1000, or even less, and so on. Thus, we can safely distinguish precious metal from base metal, and not only for gold coins.
The method we will use, and with which we will become acquainted later, is simple, reliable and non-destructive, which is extremely important, especially in numismatics. This hydrostatic method is also known as the weighing analysis, or the double weighing method.
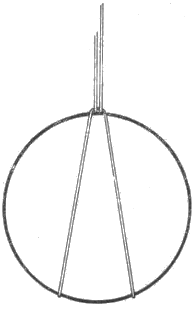
Fig. 1 Hanger for hydrostatic weighing
And as the name suggests, we cannot do without scales with a weighing accuracy of min. 0.1 g, which, along with a magnifying glass and a caliper, are among the basic tools of the numismatist.
We will also need a thin silk fishing line for tying coins, preferably of Æ 0.1 mm, and a suitable glass or other transparent container, whose dimensions allow it to be placed above the bowl of the balance, as shown in Fig. 3.
First, the coin to be examined is fitted with a hanger of the aforementioned silk fibre as shown in Figure 1. Of course, other methods of tying are possible, but do not affect the weighing result. The coin is then hung on one arm of the balance and weighed as shown in Figure 2.
The weight is marked M1 and noted. Leave the coin suspended, but slide a transparent container of water underneath it, so that the whole coin is safely submerged in the water and does not touch the sides or bottom of the container, as shown in Figure 3. The container of water can be held by hand, and if the weighing is more frequent, a suitable bridge can be made on which to place the container. Weigh the coin again while it is submerged in the water. The weight found, which we will mark M2, will be less than when weighed in air.
The difference between the two masses is the force exerted by the body immersed in the liquid according to Archimedes' law. For our purposes we may neglect the correction for the temperature of the water and the weight of the silon fibre, and consider the difference in the weights of the coin weighed in air and in water directly as its volume.
We then calculate the density using the formula:
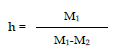
For the sake of illustration, let us take a concrete case in real numbers. A common silver five-crown from the reign of Franz Joseph I.
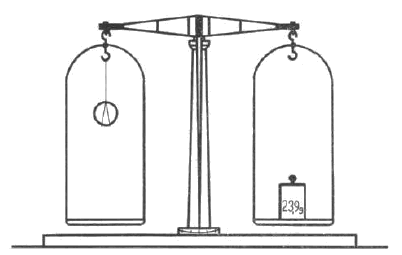
Fig. 2 Weighing in air
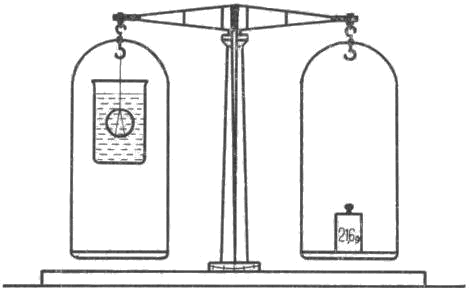
Fig. 3 Weighing in water
Weighing it in air, we find its mass M1, which is 23.9 g. The weight found by weighing in water M2 is 21.6 g. We find the density of the above five-crown by dividing the higher mass, in this case 23.9 g, by the difference of the two masses, i.e. by 2.3. The result is the density of the coin under examination, in this case h=10.3.
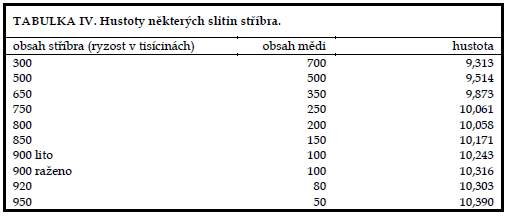
By consulting Table IV we find that this is safely a silver coin, of minimum purity 900/1000. We can certainly rule out confusion with lead, which is adjacent to silver in Density Table I, even with minimal experience.

For the sake of interest, we list in Table No. II. densities of randomly selected numismatic material, obtained by the method described, where correction for temperature and weight of the hinge has been taken into account and weighted to four decimal places.
Due to the high densities of precious metals such as platinum, gold and silver, the method described gives the most reliable results in these cases. Thus, for example, particularly in the case of binary gold-copper alloys, the determination of the density of the material can be used to determine the purity with a high degree of precision and thus safely distinguish, for example, the authenticity of gold coins (see, for example, the Table II, entries 33 and 36) without the slightest damage to the coin by comparing the density with the data in Table III.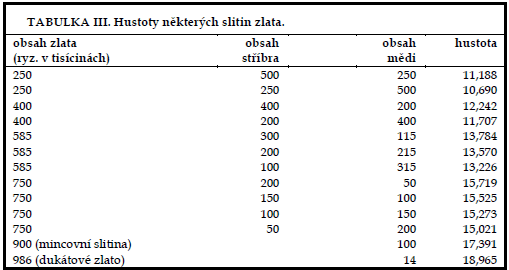
Alloys of gold with other metals, e.g. gold-nickel, gold-palladium and others are not common in coinage, therefore their densities are not given.
With careful work, we can distinguish the purities of silver alloys, so we give an overview of the densities of binary silver-copper alloys as the most common coinage metal in Table IV.
The differences in densities between stamped and cast material are less than 1%' which is negligible. The method described can therefore be used to identify coins and other objects made of base metals, as well as silver-plated or gold-plated objects (doublé, plaqué), as their density is always less than 9.
However, when determining the density by the hydrostatic method described above, it is necessary to exclude hollow objects or those whose ruggedness precludes the perfect removal of closed air bubbles on the immersed object.
If comparative numismatic material is available, which is quite common in numismatics, the probability of identifying, for example, forgeries or other imitations differing in purity increases considerably.
In conclusion of this chapter, it remains to express the conviction that the described method can save us many disappointments, sometimes even financially, even in connection with non-numismatic objects.
Do you also want to find a historical coin? Take a look at our metal detectors.
The article is included in categories:
Post
Presne tak bude to tady cele at je to prehledne pro vsechy, stale se resi co a jak cistit a toto je ucelene a rozumne zpracovane.
Pane Kotas, je chválihodné, že se chcete podělit o své poznatky s čištěním mincí, ale tento způsub, velice zlehčeně řečeno, je nešťastný. Obávám se, že v tomto případě nepomůže ani citace zroje...

Me to prijde docela jako zlodejna, proc si jsem nedal rovnou odkaz na tu knziku, kdyz je to cele jen zkopirovane :(
Oceňuji, že jsi si dal práci, ale taky mi to hlava nebere, proč to děláš. Kdyby jsi měl vlastní zkušenosti s čištěním a chtěl se podělit o originální postupy, porovnával metody apod., tak bych to právě pochopil. Ale není toto plýtvání energií?
Chtěl jsem někde začít a na to navázat srovnání uváděných metod + metod různě posbíraných např.: čištění Cu mincí pomocí Chelatonu, kyseliny citronove, kyseliny fosforecne, elektrolyzou, pouze vodou a vyvolat nad těmito postupy diskusi. Ne všichni si zmíněné informace jsou schopni dohledat, proto jsem myslel, ze tento "začatek" by mohl byt zde uveřejněný formou článků a ne pouze odkazu. Jak vidím dle reakcí zvolený postup není dle Vás asi moc šťastný, tak ještě prodiskutuji s povolanějšími a uvidím zda nepřehodnotím a nezveřejním pouze srovnání metod s odkazem na ono pdf.
V administraci máme ještě 4 díly co s nimi?
Kritizovat je snadné  ale já ocenuji tu snahu a že si s tím dal tu práci a ne všichni vědí, že je knížka od Bukvice. Tak že za mě, proč ne
ale já ocenuji tu snahu a že si s tím dal tu práci a ne všichni vědí, že je knížka od Bukvice. Tak že za mě, proč ne  třeba to pár hledačům pomůže.
třeba to pár hledačům pomůže.
Máro, dal bych je do archívu, sekce čištění. Kdo chce tak si to tam najde a kdo to číst nepotřebuje tak to ani otvítat nebude.. 

Když už nic jinýho, tak snaha by se měla ocenit, takže díky za to!
To endy: do archivu to jde automaticky. Obecně, já jsem to neznal a především 4 část je pro mě velice hodnotná. Jelikož tam mám co otestovat. 
I když je to opsaný tak není špatnej nápad dát to někam sem, pro ty co to neznají. Maximálně bych někam napsal zdroj. Taky bych potřeboval nějakej dobrej návod na čištění měďáků. Mám pár pěknej, bezcenejch kousků co bych si rád vyčistil. Naposledy jsem zkoušel natřít minci Lakostanem, max. pět minut. Pak jsem to zneutralizoval v octové vodě. Minci jsem celkem pěkně vyčistil bez poškození patiny ale bohužel při neutralizaci v octě dostala nějaký namodralý nádech  .
.
Za mne díky,rád si počkám na rozuzlení 
Zdravím všechny, jsem rád, že jsou i pozitivní názory  Zdroj je samozřejmě uveden, ale jako vždy až na konci takže v devátém článku. Nicméně kvituji připomínky ohledně testů. Sám připravím testy na měď. Porovnám výsledky čistění (kyselina citronová, kyselina fosforečná, Chelaton 3, elektrolýza, solvina, voda - tyto jsem zkoušel, případně vítám další nápady nebo pomoc aby byl materiál obsáhlejší). S ostatním čištěním nemám zkušenosti, takže rád od Vás přivítám pomoc a zapracuji do článků.
Zdroj je samozřejmě uveden, ale jako vždy až na konci takže v devátém článku. Nicméně kvituji připomínky ohledně testů. Sám připravím testy na měď. Porovnám výsledky čistění (kyselina citronová, kyselina fosforečná, Chelaton 3, elektrolýza, solvina, voda - tyto jsem zkoušel, případně vítám další nápady nebo pomoc aby byl materiál obsáhlejší). S ostatním čištěním nemám zkušenosti, takže rád od Vás přivítám pomoc a zapracuji do článků.
Elmara: druhý díl se může zveřejnit jak je, třetí dodám úpravy o testy, a každý další pak také pokud někdo nabídne své zkušenosti, které bychom mohli zapracovat.
CheGuevara: tvou pomoc rád využiji a zařadím do článku 5 (zlato + stříbro)
Přivítám návrhy i pomoc. Kontakt na mě je: riors@riska.cz skype:RiorsCZ
Tak tenhle článek vypadá tak luxusně, že musim napsat komentář dřív než ho přečtu 

No tak po přečtení článku a hlavně komentářů z toho mám rozporuplné pocity. Na jednu stranu jsem se dozvěděl něco zajímavého (ač přísně vzato ne nového, prostě opakování fyziky základní školy), na druhou stranu pokud je článek vytvořený pomocí ctrl+c - ctrl+v, nemůžu autorovi vyslovit pochvalu. Pochvalu by v tom případě zasloužil pan Bukvic. Ale já jeho knížky nečetl, takže to nemůžu posoudit.
I přesto, že knihy pana Bukvice jsou už staršího data, předpokládám, že fyzikální zákony platí dnes stejně jako tenkrát. Proto s aktuálností této části seriálu nemám nejmenší problém. Stejně tak jako s faktem, že článek čerpá z díla někoho jiného. Autor na tuto skutečnost na začátku upozorňuje. Otázkou však stále zůstává, do jaké míry je text upraven a do jaké míry zůstal v původním znění. Dále bych očekával, že zdroje budou uvedeny na konci každé části, nikoliv až na samém konci seriálu. Pak by to totiž mohlo vypadat (při nějaké nešťasté formulaci), že z daných zdrojů čerpá pouze ta poslední část a ne i všechny předchozí.
Pokud jsou v dalších částech skutečně i autorovy osobní poznatky, rád bych si přečetl seriál celý. Pokud ne, tak děkuji mnohokrát, spokojím se s tou výše zmiňovanou knihou 
Ještě bych rád zareagoval na ty, co píší, že snaha se cení. To je právě jádro pudla, jak moc se autor snažil sám a jak moc pouze kopíroval. Protože kdyby to měly být pouze zkopírované části nějaké knihy, tak to moc snahy nevyžaduje.  Ale samozřejmě pokud to budou rady z knihy konfrontované s vlastními zkušenostmi, tak je to naprosto v pořádku, nic jiného bych ani nechtěl. Velmi těžko se dají na tomto poli vymýšlet vlastní originální metody, tím spíše, když je autor začátečník. Naopak porovnání nějaké teorie v knize s praxí (hlavně ty fotky!) bych jenom uvítal, protože tím může autor řadě lidí ulehčit práci při hledání těch správných metod k čištění.
Ale samozřejmě pokud to budou rady z knihy konfrontované s vlastními zkušenostmi, tak je to naprosto v pořádku, nic jiného bych ani nechtěl. Velmi těžko se dají na tomto poli vymýšlet vlastní originální metody, tím spíše, když je autor začátečník. Naopak porovnání nějaké teorie v knize s praxí (hlavně ty fotky!) bych jenom uvítal, protože tím může autor řadě lidí ulehčit práci při hledání těch správných metod k čištění. 
Supr článek. Až na nejspíš neuvědomělé chybky rozhodně palec  rozhodně pokračuj a věřím že to busde stát za to
rozhodně pokračuj a věřím že to busde stát za to 
"Na... moralisty... kašli." Nač si otravovat život morálkou, hlavně, že mám z věci prospěch... výborně pane Itale.
Všechny výrazně negativní komentáře byly napsány před Riorsovýn vysvětlením, takže bych se do nich nenavážel jakožto do všeználků atd., protože v době, kdy vznikaly, byly k věci a jejich autoři měli naprostou pravdu. Jinak já jsem nekomentoval článek, ale vaše povzbuzování k chování ve smyslu: "Autorská práva? Pchá, tohle je internet..." Oním prospěchem jsem nemyslel prospěch autora článku, nýbrž jeho čtenářů, a co se mého výkřiku týče (že byl zbytečný si nemyslím; že byl marný, s tím počítám) - ano, byl myšlen vážně.
Ital1981: Po tomhle komentáři, který ignoruje vše mnou napsané, jsem si musel tu knížku najít a porovnat to. Celá tahle část je slovo od slova okopírovaná. A to mi tady připadá zcela zbytečné. Stačilo přece před svůj vlastní text přidat poznámku ve smyslu, že pro úplné začátečníky je vhodná kniha Jan Bukvic: Čištění a konzervování mincí. Ta druhá část je úplně to samé, prostě zkopírovaný text z knihy. Akorát vynechal dost dlouhou část o puncování. Jak jsem napsal výše: vlastní zkušenosti uvítám, ale okopírovaná kniha mě fakt nezajímá 
Jaký zdroj? Si normální Itale? Ty si myslíš, že když konmpletně okopíruješ něčí dílo, něčí knížku i s obrázkama a napíšeš tam zdroj, že jsi jako z obliga? Říkají Ti něco autorská práva?
Mě samotnému tady zveřejnil článek, který jsem psal pro časopis Mince a Bankovky a nějaký šulibrk udělal jenom CTRL+V a CTRL+C a napsal tam pod to zdroj: "internet". To si jako myslíš že to je naprosto normální? Ne opravdu to není normální a rozhodně nestačí, že tam uvede zdroj, když to kompletně zkopíruje i s obrázkama! Zdroj se uvádí, pokud cituješ přímo něčí knihu - ale jen její odstavec, nikoliv celou knihu! Takže si laskavě vyndejte hlavy ze zadku, než se to začne řešit právně, jelikož toho už mám vážně dost.
Pokud tady uživatelé nemají dostatek inteligence aby vytvořili vlastní články s pomocí takovéto a podobné literatury (s pomocí, ne když to komplet zkopírují) tak to je holt asi problém. Tady si kopíruje kdo chce co a omluva "ono to stejně koluje na internetu" nebo "je to lepší než kdyby seděl u televize" opravdu není žádný právní argument. Představte si, že byste třeba 10 let tvořili nějakou knihu a pak by přišel nějaký trouba a celé to hodil na net a uvedl by tam akorát - toto napsal "autor". Věřím že by Vám to nejenom zvedlo mandle a otevřelo kudlu v kapse. Ne, toto rozhodně není v pořádku v žádném směru.
ridge: Přesně tak.